Omnix Medical is honored to have earned a recent grant award from the Israel Innovation Authority to support continued development of new and effective lifesaving drugs to combat the spread of antimicrobial resistance.

— Existing and new investors joining forces to support the accelerated development of Omnix´s novel, first-in-class antimicrobials pipeline — Funding underlines urgent global need for
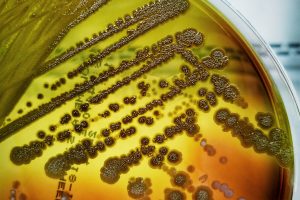
Omnix Medical tested its novel antimicrobial peptide, OMN51, on clinical isolates of multi-drug-resistant Pseudomonas aeruginosa collected from cystic fibrosis patients, in collaboration with Beilinson – Rabin Medical Center.

“Antibacterial agents active against Gram‑Negative Bacilli in Phase I, II, or III Clinical Trials,” by David L. Paterson, surveys nearly 50 investigational therapeutics—28 small‑molecule antibiotics and 21 non‑traditional modalities—now in the clinic. The review highlights
Omnix Medical Ltd. is committed to making its website and all its electronic information easier to use and more accessible for people with disabilities. The company’s website was created and/or updated according to the provisions of the Equal Rights for People with Disabilities Law, 5748-1998, and the delegated legislation stemming from it, and it fully complies with the W3C Web Content Accessibility Guidelines 2.0, AA level.
Omnix Medical Ltd. will continue its efforts to constantly improve the accessibility of its website and information in the belief that it is our collective moral obligation to allow seamless, accessible, and unhindered use also for those of us with disabilities.
 This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 966627 (OMNIX MEDICAL).
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 966627 (OMNIX MEDICAL).