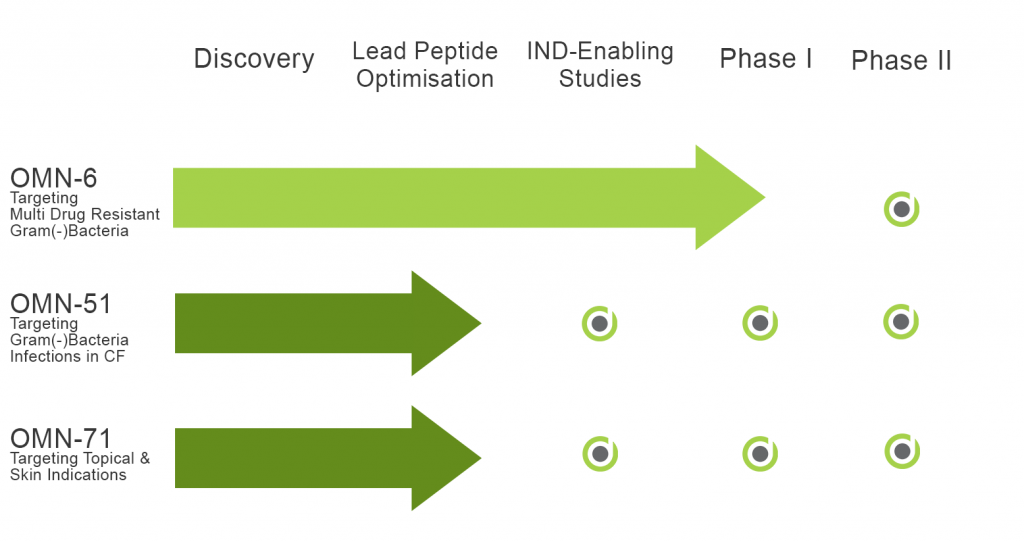
Omnix Medical is pleased to announce the addition of two new peptide families – OMN50 and OMN70.
At Omnix, we are working hard to build our clinical pipeline with new peptide-based drugs that address broad unmet medical needs.
Using Omnix drug discovery platform to screen a wide-ranging array of peptides originating from marine organisms, insects and frogs, we identified these two new peptide families that demonstrate a great potential in targeting hard to treat bacterial pathogens.
Two molecules, OMN51 and OMN71, have been advanced to Lead Optimization and Formulation stages of their development:
OMN51 is a new antimicrobial peptide (AMP) targeting Gram-negative bacteria like P. aeruginosa which causes recurrent and persistent infections in patients with Cystic Fibrosis (CF). Cycles of infection and hospitalization promote the development of resistance that significantly reduces treatment solutions.
OMN71 is a new antimicrobial peptide (AMP) targeting bacteria involved with skin infections in patients with Acne or Atopic-Dermatitis. When infections of the skin involve MRSA or P. Acnes high levels of resistance are present and treatment becomes hard and prolonged.
Intensive and comprehensive preclinical research is being conducted at Omnix Medical Laboratories to bring OMN51 and OMN71 to clinical development in parallel with the filing of appropriate patent applications.
The Company’s lead compound, OMN6, is a first-in-class antimicrobial peptide for the treatment of life-threatening hospital acquired infections caused by Gram-negative bacteria. OMN6 has been found to be highly effective on multidrug-resistant Acinetobacter baumannii (AB), especially Carbapenem-resistant AB (CRAB), the #1 priority pathogen according to the WHO/CDC.
Recently, Omnix Medical initiated a Phase I clinical trial testing our lead molecule, OMN6, in a randomized, double-blind, placebo-controlled, single ascending dose trial assessing safety, tolerability, and pharmacokinetics in healthy subjects. The trial is conducted in Groningen, The Netherlands and results are expected by Q4 2022.
To learn more about the Omnix discovery pipeline, contact us at info@omnixmedical.com.

 This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 966627 (OMNIX MEDICAL).
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 966627 (OMNIX MEDICAL).