PIPELINE
Omnix Medical’s pipeline maintains three major programs.
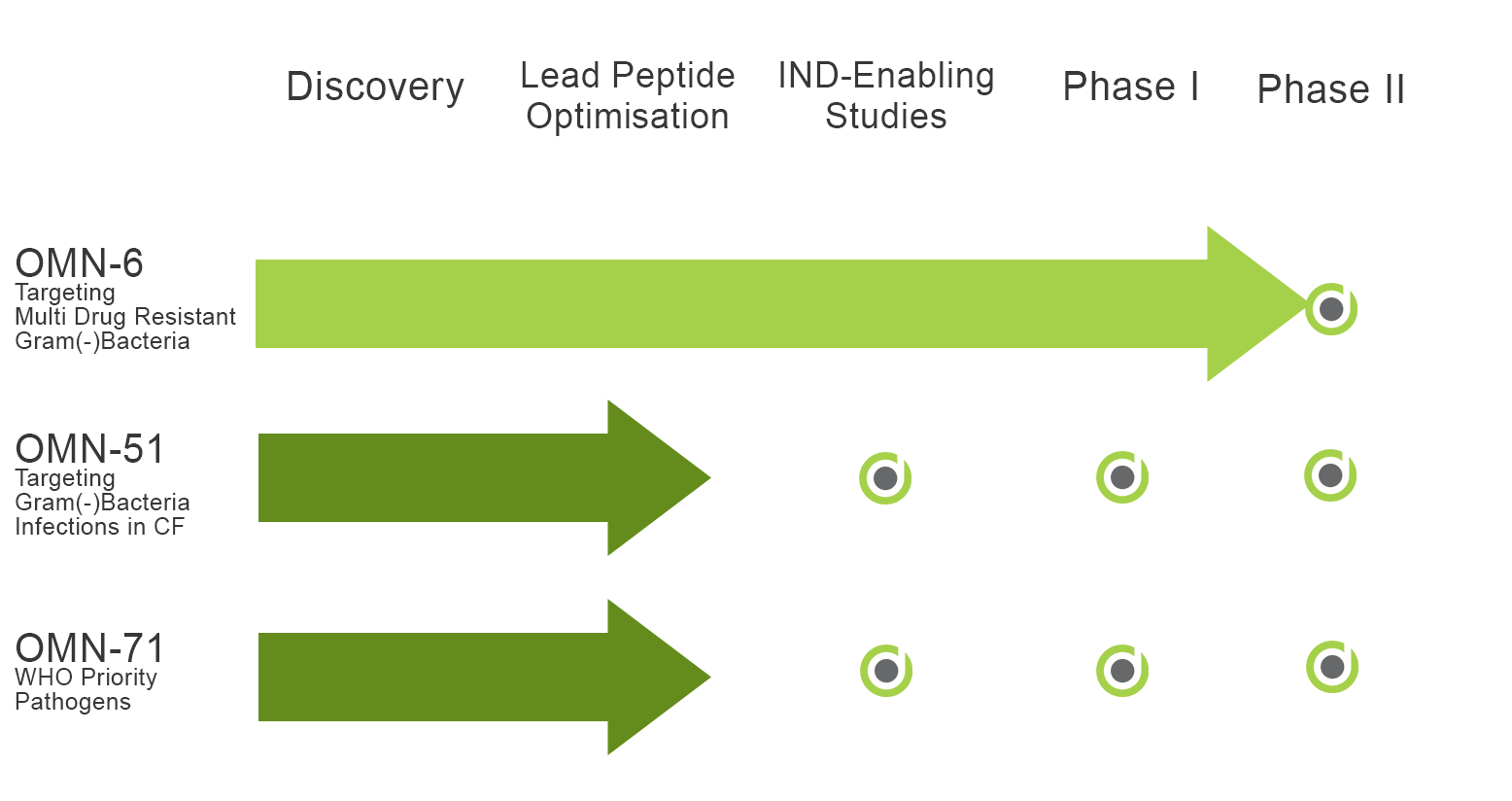
The Company’s lead compound, OMN6, has been found to be safe, stable, and highly effective against multidrug-resistant Acinetobacter baumannii (AB), especially Carbapenem-resistant AB (CRAB), which is the #1 priority pathogen according to the WHO and CDC. The company successfully completed a Phase 1 trial with OMN6, demonstrating a favorable pharmacokinetic (PK) profile with no severe or adverse events. Considering OMN6’s powerful antimicrobial activity, lack of resistance, and safety at clinical exposure levels demonstrated in the Phase 1 clinical study, OMN6 is well-positioned to become a first-line treatment for severe or life-threatening infections involving multidrug- and carbapenem-resistant AB.
The lack of new and available treatment options, combined with the global spread of antimicrobial resistance, presents a low-risk to high-benefit ratio for the patient population with HABP or VABP. This strongly supports the progression of OMN6 into a Phase 2 clinical trial. Omnix has recently initiated an FDA-approved Phase 2 trial targeting hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) caused by the Acinetobacter baumannii complex (ABC).
OMN-51 is a new antimicrobial peptide (AMP) targeting Gram-negative bacteria like P. aeruginosa which cause recurrent and persistent infections in patients with Cystic Fibrosis (CF), Non–Cystic Fibrosis Bronchiectasis (NCFB) and COPD. Cycles of infection and hospitalization promote the development of resistance that significantly reduces treatment solutions.
OMN-71 is a new antimicrobial peptide (AMP) targeting bacteria involved in skin and topical indications. Skin infections often involve bacteria with high levels of resistance, making treatment difficult and prolonged.
Intensive and comprehensive preclinical research is being conducted at Omnix Medical Laboratories to bring OMN51 and OMN71 to clinical development in parallel with the filing of appropriate patent applications.
 This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 966627 (OMNIX MEDICAL).
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 966627 (OMNIX MEDICAL).