TECHNOLOGY

Extensive and widespread use of antimicrobial drugs led to the emergence of resistant strains of microorganisms. These microorganisms are no longer susceptible to the currently available antimicrobial drugs. Today, bacteria are acquiring resistance to all available antibiotics at a much faster rate than the introduction of new drugs and are posing a serious and imminent threat to public health. Moreover, since their discovery, antibiotics have served as the cornerstone of modern medicine. Antimicrobial Resistance (AMR) has led to the emergence of untreatable superbugs that now threaten the basis of modern medicine.
State-of-the-art approaches to treat MDR infections are desperately needed.
Antimicrobial Peptides (AMPs) are part of the innate immune response found among all classes of life. The ability of natural occurring antimicrobial peptides to efficiently and selectively kill bacteria has endured for millions of years of evolution and is the inspiration at the core of our technology.
AMPs have membrane perturbing abilities, allowing them to disrupt target membranes and facilitate the depletion of the bacterial electrochemical potential or permeate into the infectious agent to reach cytoplasmic targets. For the first time, our proprietary innovation allows AMPs to become drugguble peptides. We have applied our platform technology predominantly to AMPs from the Cecropin peptide family, and by implementing a breakthrough bioengineering strategy, we have overcome the two main barriers that stand in the way of using antimicrobial peptides as effective therapeutic agents – stability and selectivity. We are able to produce selective, degradation-resistant peptides that are highly potent by both synthesis and biochemical engineering approaches.
We have based our design on insect host defense peptides due to their remarkable potency and pathogen-selective properties, and we have created cyclic constructs of the most efficacious AMPs. These cyclic constructs represent higher stability while maintaining bioactivity. The end results are novel, druggable and lifesaving pharmaceuticals.
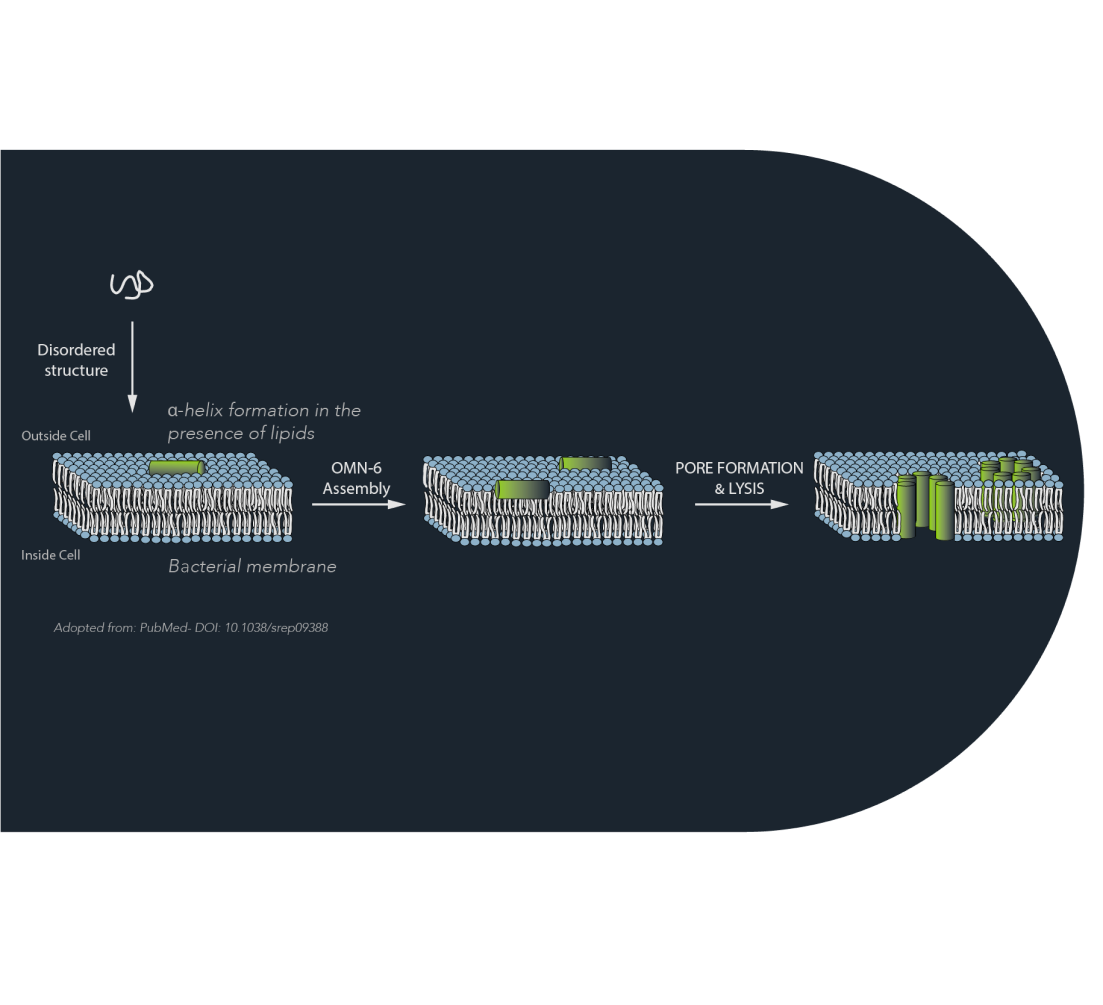
A representation of the Mechanism of Action employed by OMN6. Several OMN6 monomers target bacterial membranes, line on the outer surface of the membrane and together form an aquouse pore
The Unique Mechanism of Action Avoiding Resistance
The Mechanism of Action (MoA) employed by OMN6, and all Omnix peptides, differs from the mechanism by which most currently-used antibiotics exert their antibacterial activity. OMN6 exerts a rapid bactericidal effect in contrast to most antibiotics which are bacteriostatic.
The target of OMN6 is the bacterial membrane in contrast to small molecule based agents which bind to a specific enzyme or bacterial site. OMN6 MoA is effective regardless of the bacteria genotype or resistance phenotype, thus, is by orders-of-magnitude, less likely for mutation driven development of resistance, recrudescence, or tolerance.
The peptide’s unique, fast acting MoA constitutes an important advantage of the technology developed by Omnix over existing technologies and may be considered a new class of antimicrobial drugs.
AMPs selectivity and the ability to distinguish between the membranes of host cells and those of bacteria are based on membrane lipid composition, namely the presence of Cholesterol, and on the net electric charge of the outer bacterial membrane, which is a key factor. Briefly, bacterial membranes are very well organized. They lack Cholesterol in them and are charged with a negative electric charge. Eukaryotic membranes harbor Cholesterol that conveys too much fluidity which inhibits the peptides activity. Eukaryotic membranes also have neutral net charge so that the peptides affinity for them is very low. Interactions with membranes of bacteria will trigger the AMP to change structure, inducing membrane perturbing effects. Because of this property, our AMPs can be designed to be harmless to hosts but deadly to the targeted pathogen.
 This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 966627 (OMNIX MEDICAL).
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under Grant Agreement No 966627 (OMNIX MEDICAL).